Aptamer-based Multivariate Index Assay (AptoMIA)
As it is difficult to diagnose the diseases caused by various factors, such as cancer, with a single biomarker, the diagnostic paradigm is shifting to the Aptamer-based Multivariate Index Assay (AptoMIA) that measures multiple biomarkers to increase sensitivity and specificity.
Since 2006, the U.S. FDA has announced the criteria for determination in expectation of medical usefulness and research facilitation of the In-vitro Diagnostic Multivariate Index Assay (IVDMIA) and the South Korea MFDS(formerly known as the Ministry of Food and Drug Safety or KFDA) also published the guidelines on the approval and review of IVDMIA medical devices in 2016.
Aptamer Sciences has established and operated Aptamer-based Multivariate Index Assay technologies, which simultaneously measure multiple biomarker proteins contained in the blood by taking advantage of the high specificity of aptamers and providing diagnostic information based on the algorithm-based analysis. Aptamers (nucleic acid materials) are capable of detecting a trace amount of target proteins owing to their ability of PCR amplification, indicating that they are more competitive than antibody technologies.
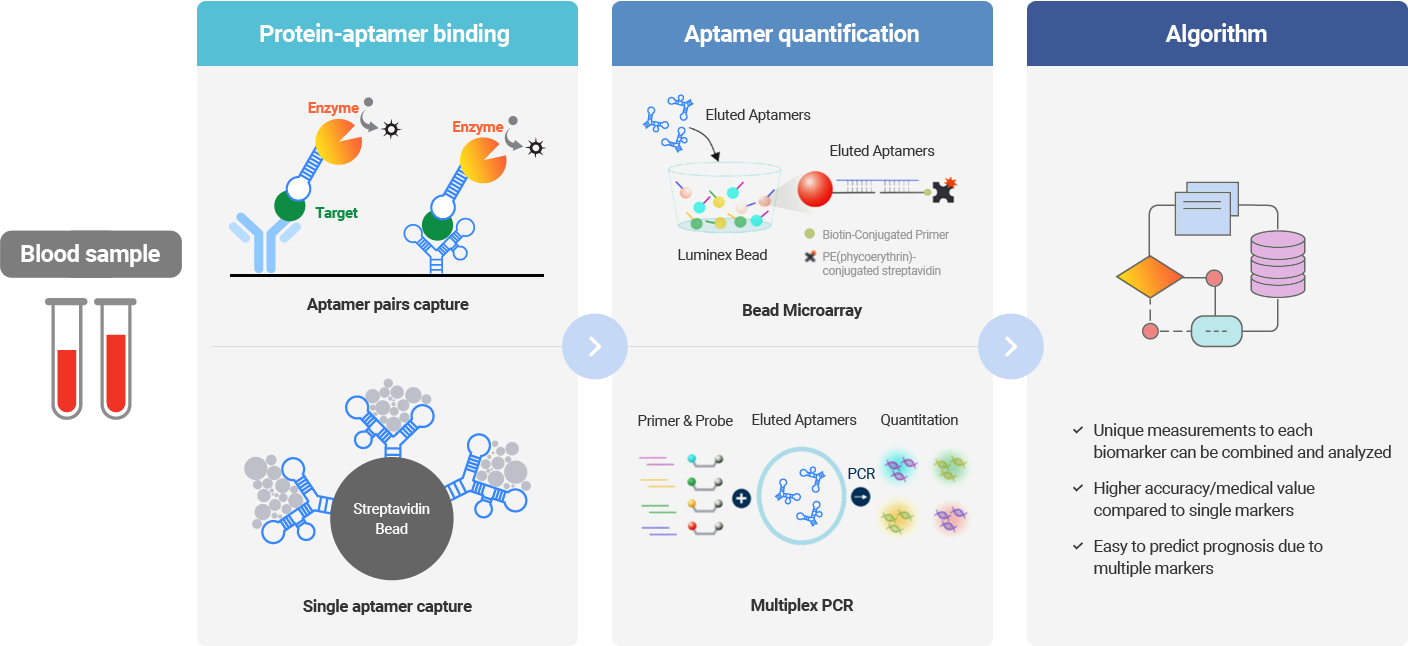
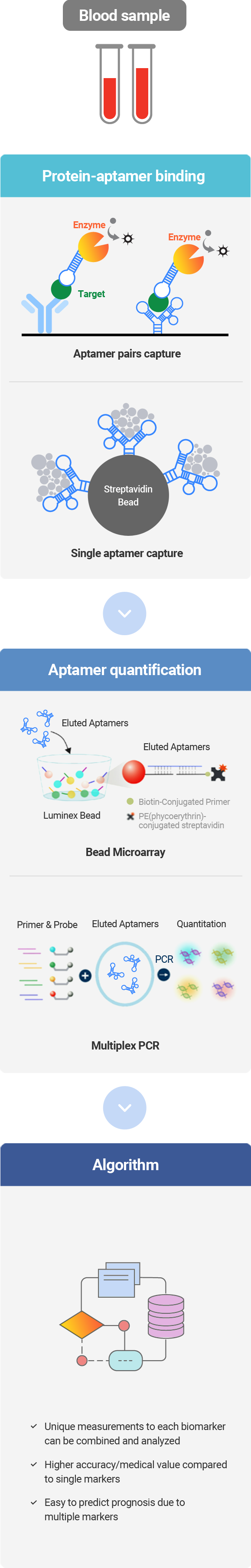
Aptamer Sciences successfully developed AptoDetect™-Lung, a product that can diagnose lung cancer in its early stage using AptoMIA technology. AptoDetect™-Lung has been subject to the quantitative analysis of 7 types of proteins contained in the blood, and it is the world's first aptamer-based diagnostic product that has obtained product approval from the MFDS.

