AptoDetect™-Lung
Lung cancer is the most common carcinoma with the highest incidence rates and the urgent need for early diagnosis.
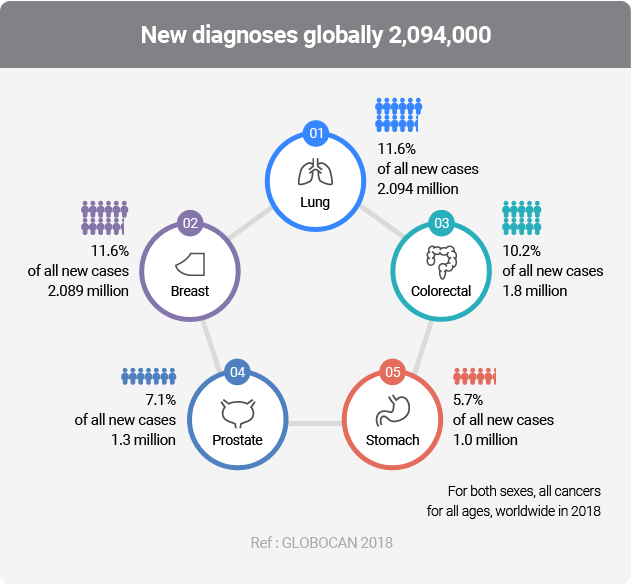
AptoDetect™-Lung is a lung cancer diagnostic kit developed by using our unique diagnostic platform AptoMIA technology, and it is the world’s first approved aptamer-based diagnostic product.
Product Overview
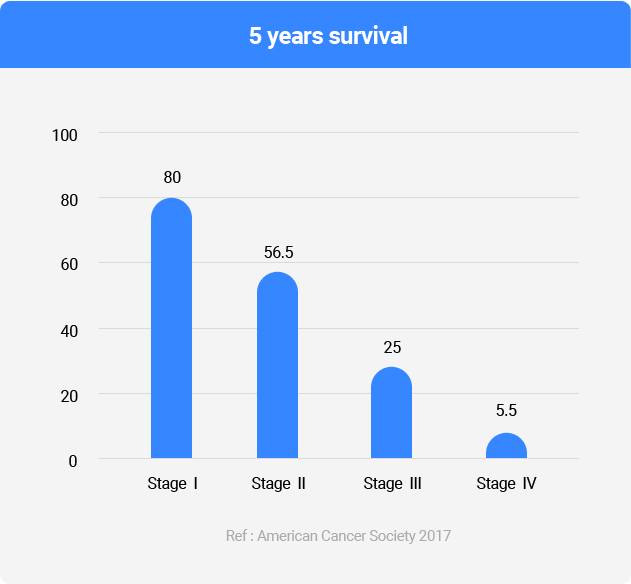
- Measure 7 types of proteins in the blood and provide information on lung cancer risk scores through an algorithm
- Provide early diagnostic information to high-risk cancer groups such as patients with indeterminate pulmonary nodules and long-term smokers, etc.
- Registered the patent in South Korea and submitted the patent application overseas (September 2015)
- Obtained the approval of Class 3 in-vitro diagnostics (IVD) medical devices from the Ministry of Food and Drug Safety (September 2017)
- Obtained the CE Certification (August 2018)
- Secured the production facilities with KGMP and ISO13485 Certification (located in Pohang)
User Instructions
AptoDetect™-Lung measures a total of 7 types of proteins, which is consisted of four types of cancer growth-related proteins (EGFR1, MMP7, CA6, and KIT) and three types of proteins related to immunity (CRP, C9, and SERPINA3), in a few drops of blood (5 uL) and provides information on lung cancer risk scores through its own algorithm calculations.
-
- STEP 01
- Blood Collection
-

- STEP 02
- Aptamer Assay (7 Biomakers)
-
- STEP 03
- Luminex Detection
-
- STEP 04
- Algorithm
-
- STEP 05
- Report
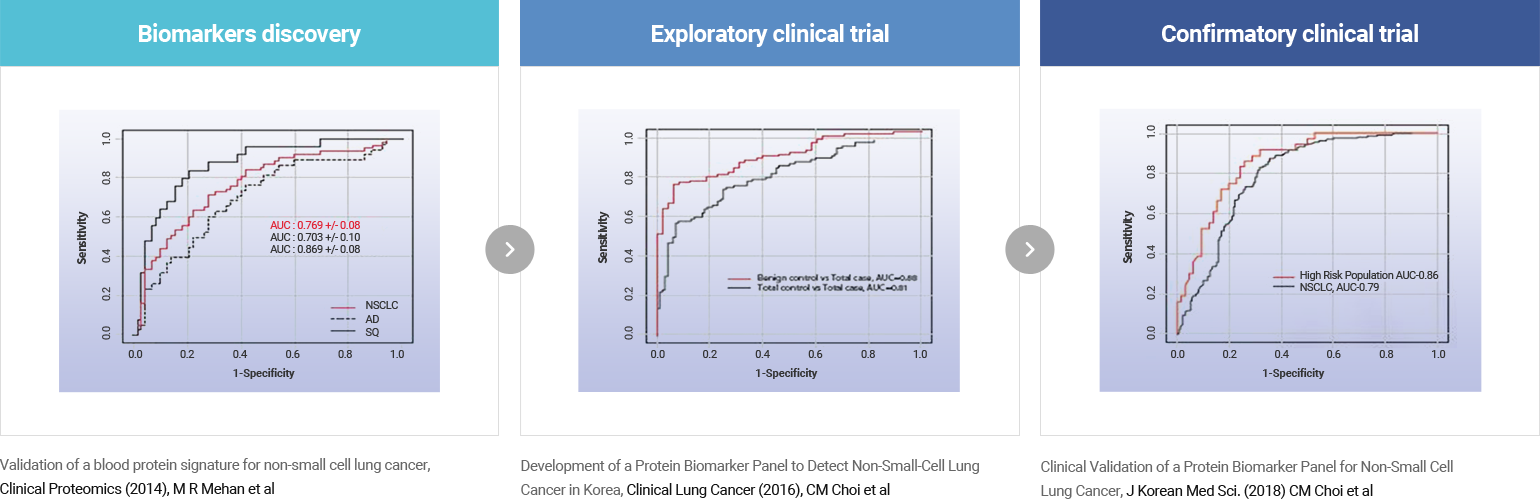
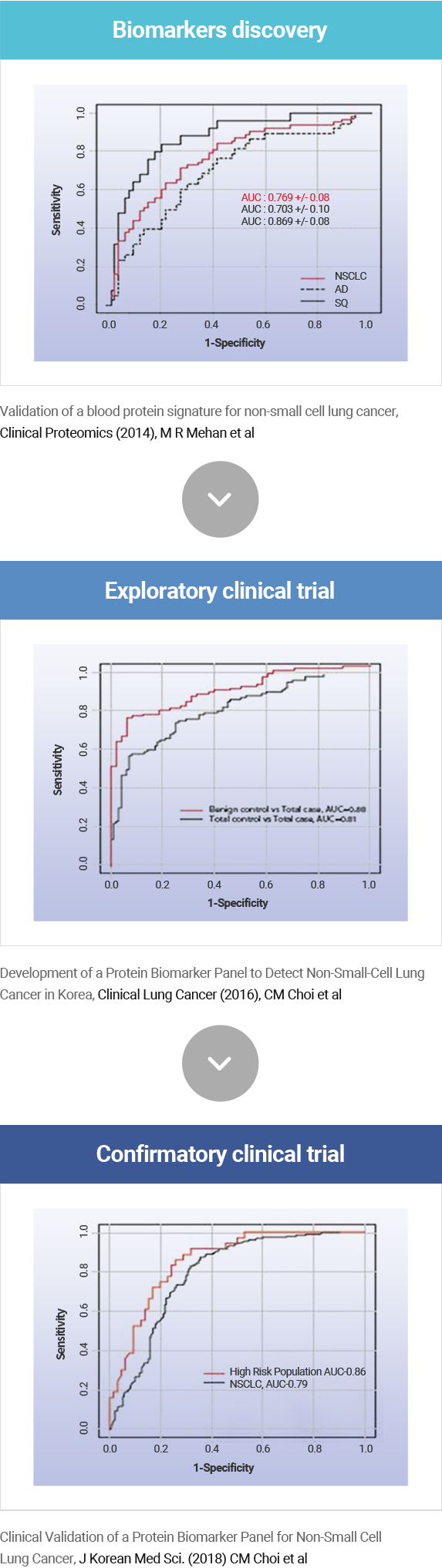
Clinical Performance Verification
AptoDetect™-Lung was co-developed and clinically validated in collaboration with POSTECH, Asan Medical Center, and SomaLogic, Inc.
Commercialization
In South Korea, we are planning to make entries in medical examination centers and hospitals through an improved nHTA(New Health Technology Assessment) system.
For overseas markets, we are undertaking clinical trials in cooperation with local partners in China and Singapore, and the products will be released in the local market when approved by the competent authority.

