Therapeutic
Cutting-edge aptamer-based technology is being harnessed to drive innovation in the development of new drugs, primarily targeting medical conditions with significant unmet needs. Built upon the BIFAP (Bi-Functional Aptamer) platform, which capitalizes on aptamer complex capabilities including target selectivity, specificity, and activity modulation, the focus lies on cultivating a novel pharmaceutical pipeline. This pipeline aims to deliver a diverse range of therapeutic agents, including small molecules, antibodies, and nucleic acids, with a specific emphasis on addressing both cancer and neurological disorders.
| Type | Code name | Target | Indication | Lead discovery | Lead Optimization | Pre-clinical | Clinical Trial | ApDC | AST-201 | GPC3 | Solid tumors (HCC) |
|---|
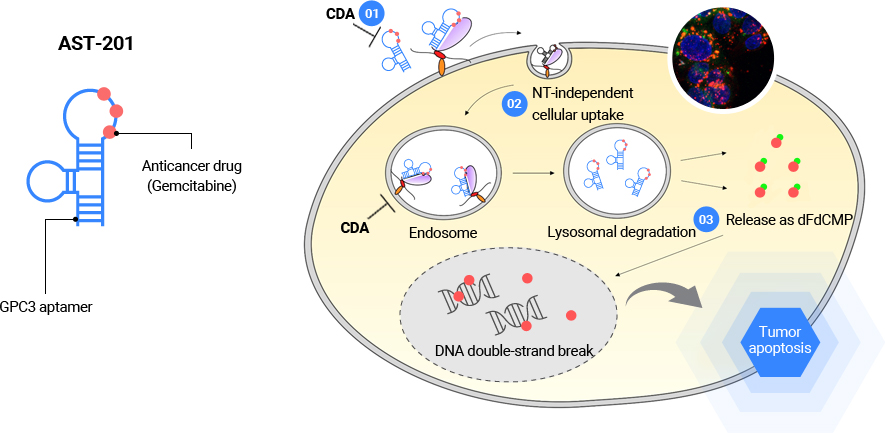
GPC3 is a glycoprotein on the surface of the cell membrane, and it is known as a factor that regulates cell division and growth. GPC3 is over-expressed in a number of cancers including liver cancer, and is attracting attention as a major target for cancer treatment. AST-201 is an aptamer-drug conjugate (ApDC) incorporating an anti-cancer agent in an aptamer with high selectivity and specificity for GPC3. GPC3-mediated intracellular influx increases the efficiency of drug delivery, protects the activity of the incorporated anti-cancer agent, gemcitabine, and allows the effective delivery to tumor tissues. AST-201 which is designed in a form that can completely overcome the mechanisms of drug resistance against gemcitabine in cancer tissues and cells, lowers the possibility of reduced effects of anti-cancer drugs. In addition, unlike antibodies or other protein conjugates, AST-201, as a nucleic acid-based material, has secured excellent tissue penetrability, allowing effective solid cancer treatments.
Tumor targeting and mechanisms for overcoming the gemcitabine resistance of tumors by AST-201
-
- 01
- Protection of Gemcitabine from intracellular/extracellular cytidine deaminase (CDA)
-
- 02
- Resisting human Nucleoside Transporter (hNT) mutations of cancer cells with introducing hNT independent cells
-
- 03
- Fast onset of pharmacological actions through bypassing the activation step of gemicitabine mediated by deoxycytidine kinase (dCK) and direct releasing active gemcitabine (dFdCMP) by lysosomal degradation
- Publication & IP
- Jun Young Park, et al. (2020) Targeted therapy of hepatocellular carcinoma using gemcitabine-incorporated GPC3 aptamer. Pharmaceutics, 12(10):985.
- Glypican-3 specific modified aptamer and uses thereof (Application 10-2154683, PCT/KR2020/015536).
| ApDC | AST-202 | CD25 | Blood tumors & Antitumor immunity |
|---|
Regulatory T cells (Tregs), which have immunosuppressive functions, have high tumor infiltration and a low ratio of effector T cells (Teffs) to Tregs within a tumor is associated with poor prognosis in various solid tumors. Conversely, a higher ratio of Teffs to Tregs within a tumor has shown valid results in immunotherapy. CD25 (IL-2RA) is an alpha chain of IL-2 high-affinity receptors, which is expressed at high levels on the surface of Tregs but is absent or expressed at low levels on the surface of Teffs. Therefore, as a therapeutic approach to treating solid tumors, CD25 is considered one of the potential protein targets to eliminate tumor-infiltrated Tregs. In addition, high expression of CD25 has been reported in a number of hematologic malignancies. AST-202, an aptamer-based targeted drug delivery molecule for immunotherapy, is designed to deplete Tregs in tumor tissue by conjugating a cytotoxic drug to a CD25-targeting aptamer with high target selectivity and specificity, resulting in immune activation to enable effective solid tumor treatment and direct treatment of overexpressed CD25 in blood cancers.
| ApDC | - | Trop2 | Solid tumors |
|---|
Trop-2, a transmembrane protein which is over-expressed in various tumors, is a key regulator of cancer characteristics such as cell growth and proliferation, attracting much attention as a target for targeted therapeutics. Trop-2 targeted treatment which is being jointly developed with PINOTBIO is an aptamer-drug conjugate (ApDC) produced by conjugating a potent cytotoxic anticancer agent to an aptamer that binds to Trop-2. As PINOTBIO’s unique linker-payload technology is grafted onto an aptamer with high target selectivity, the maximization of drug safety and anticancer effects through target-selective drug delivery is expected. Target-delivered drugs are introduced into cells by receptor-mediated endocytosis and decomposed by lysosomes to release the drug. Also, the drug is designed to be released in an acidic environment outside cancer cells, so its anti-cancer effects can be expected even in surrounding cancer cells and in the tumor microenvironment where drug delivery is difficult.
| ApRC | - | Trop2 | Solid tumors |
|---|
EGFRvIII is a mutant protein that promotes cancer cell growth by making its expression only in cancer cells and cancer stem cells as an epithelial cell growth factor receptor mutant. EGFRvIII is specifically present in 28-30% of glioblastomas, and as most positive patients have a poor prognosis, EGFRvIII is attracting attention as a major target in the glioblastoma field where there is no targeted treatment. The treatment for glioblastoma that is being jointly developed with PINOTBIO is an aptamer-drug conjugate (ApDC) produced by conjugating a potent cytotoxic anticancer drug to an aptamer that binds to EGFRvIII. As a result of applying the isotope-labeled EGFRvIII aptamer to a xenograft tumor model, the excellent tumor targeting performance of the aptamer itself was found. Also, as using PINOTBIO’s unique linker-payload technology combines with aptamers with high target selectivity, the maximization of drug safety and anticancer effects through target-selective drug delivery can be expected. Target-delivered drugs are designed in a way that they can be released in an acidic environment inside and outside of cancer cells, so their anti-cancer effects can be expected even in surrounding cancer cells and in the tumor microenvironment where drug delivery is difficult.
| ApIS | - | Undisclosed | Antitumor immunity |
|---|
EGFRvIII is a mutant protein that promotes cancer cell growth by making its expression only in cancer cells and cancer stem cells as an epithelial cell growth factor receptor mutant. EGFRvIII is specifically present in 28-30% of glioblastomas, and as most positive patients have a poor prognosis, EGFRvIII is attracting attention as a major target in the glioblastoma field where there is no targeted treatment. The treatment for glioblastoma that is being jointly developed with PINOTBIO is an aptamer-drug conjugate (ApDC) produced by conjugating a potent cytotoxic anticancer drug to an aptamer that binds to EGFRvIII. As a result of applying the isotope-labeled EGFRvIII aptamer to a xenograft tumor model, the excellent tumor targeting performance of the aptamer itself was found. Also, as using PINOTBIO’s unique linker-payload technology combines with aptamers with high target selectivity, the maximization of drug safety and anticancer effects through target-selective drug delivery can be expected. Target-delivered drugs are designed in a way that they can be released in an acidic environment inside and outside of cancer cells, so their anti-cancer effects can be expected even in surrounding cancer cells and in the tumor microenvironment where drug delivery is difficult.
| ApDC | - | Undisclosed | Solid tumors (SCLC) |
|---|
EGFRvIII is a mutant protein that promotes cancer cell growth by making its expression only in cancer cells and cancer stem cells as an epithelial cell growth factor receptor mutant. EGFRvIII is specifically present in 28-30% of glioblastomas, and as most positive patients have a poor prognosis, EGFRvIII is attracting attention as a major target in the glioblastoma field where there is no targeted treatment. The treatment for glioblastoma that is being jointly developed with PINOTBIO is an aptamer-drug conjugate (ApDC) produced by conjugating a potent cytotoxic anticancer drug to an aptamer that binds to EGFRvIII. As a result of applying the isotope-labeled EGFRvIII aptamer to a xenograft tumor model, the excellent tumor targeting performance of the aptamer itself was found. Also, as using PINOTBIO’s unique linker-payload technology combines with aptamers with high target selectivity, the maximization of drug safety and anticancer effects through target-selective drug delivery can be expected. Target-delivered drugs are designed in a way that they can be released in an acidic environment inside and outside of cancer cells, so their anti-cancer effects can be expected even in surrounding cancer cells and in the tumor microenvironment where drug delivery is difficult.
Early Diagnosis
Aptamer Sciences launched the world's first early diagnosis kit for lung cancer with AptoMIA-based multivariate index assay technology and is expanding the development of diagnostic kits for various diseases in stages through research cooperation with hospitals.
| Product | Biomarker development | Development of multivariate index assay | Confirmatory clinical trial | RA | Note | Aptodetect™-lung |
Diagnosis Support * Manufacturing license for medical device (Class III) from the Ministry of Food and Drug Safety (No. 17-784) |
|---|
Lung cancer
Lung cancer is the deadliest cancer worldwide.
When cancer is detected at an early stage and coupled with appropriate treatment, the chance of survival rate is higher.
But, the current imaging modalities such as chest X-RAY, CT scans have problems with a very high false-positive rate.
When using Aptamer Sciences' non-invasive lung cancer diagnosis product as an auxiliary diagnosis, you can improve the accuracy by more than 20%.
Early diagnosis
of lung cancer,
AptoDetect™-Lung
AptoDetect™-Lung, an aptamer-based multivariate index assay, provides information on the risk score of lung cancer (high/low risk)
by measuring '7' kinds of proteins in a patient's blood and analyzing them with an in-house developed algorithm.
Main Features
- Providing information on lung cancer risk score (0~10 score, and cut-off 5)
- Using a classifier consisting of 7 biomarkers (C9, CA6, EGFR1, MMP7, KIT, SERPINA3, and CRP)
- Quantitative analysis with aptamer-based bead microarray technology (using Luminex equipment)
- Clinical Performance: Sensitivity (75%), Specificity (92%)
- Ministry of Food and Drug Safety approval (Sep. 2017), CE certification (Aug. 2018)

